History
The term "photovoltaic" comes from the Greek φώς (phos) meaning "light", and "voltaic", meaning electrical, from the name of the Italian physicist Volta, after whom the measurement unit volt is named. The term "photo-voltaic" has been in use in English since 1849.[1]
The photovoltaic effect was first recognized in 1839 by French physicist A. E. Becquerel. However, it was not until 1883 that the first solar cell was built, by Charles Fritts, who coated the semiconductor selenium with an extremely thin layer of gold to form the junctions. The device was only around 1% efficient. Russell Ohl patented the modern solar cell in 1946 (U.S. Patent 2,402,662, "Light sensitive device").
Sven Ason Berglund had a prior patent concerning methods of increasing
the capacity of photosensitive cells. The modern age of solar power
technology arrived in 1954 when Bell Laboratories, experimenting with semiconductors, accidentally found that silicon doped with certain impurities was very sensitive to light.
This resulted in the production of the first practical solar cells
with a sunlight energy conversion efficiency of around 6 percent. The
first spacecraft to use solar panels was the US satellite Vanguard 1, launched in March 1958 with solar cells made by Hoffman Electronics. This milestone created interest in producing and launching a geostationary communications satellite,
in which solar energy would provide a viable power supply. This was a
crucial development which stimulated funding from several governments
into research for improved solar cells.
In 1970 the first highly effective GaAs heterostructure solar cells were created by Zhores Alferov and his team in the USSR. [2][3][4]
Metal Organic Chemical Vapor Deposition (MOCVD, or OMCVD) production
equipment was not developed until the early 1980s, limiting the ability
of companies to manufacture the GaAs solar cell. In the United States,
the first 17% efficient air mass zero (AM0)
single-junction GaAs solar cells were manufactured in production
quantities in 1988 by Applied Solar Energy Corporation (ASEC). The
"dual junction" cell was accidentally produced in quantity by ASEC in
1989 as a result of the change from GaAs on GaAs substrates to GaAs on
Germanium (Ge) substrates. The accidental doping of Ge with the GaAs
buffer layer created higher open circuit voltages, demonstrating the
potential of using the Ge substrate as another cell. As GaAs
single-junction cells topped 19% AM0
production efficiency in 1993, ASEC developed the first dual junction
cells for spacecraft use in the United States, with a starting
efficiency of approximately 20%. These cells did not utilize the Ge as
a second cell, but used another GaAs-based cell with different doping.
Eventually GaAs dual junction cells reached production efficiencies of
about 22%. Triple Junction solar cells began with AM0 efficiencies of approximately 24% in 2000, 26% in 2002, 28% in 2005, and in 2007 have evolved to a 30% AM0 production efficiency, currently in qualification. In 2007, two companies in the United States, Emcore Photovoltaics and Spectrolab, produce 95% of the world's Triple Junction solar cells which have a commercial efficiency of 38%. In 2006 Spectrolab's cells achieved 40.7% efficiency in lab testing.[5]
[edit] Three generations of solar cells
Solar Cells are classified into three generations which indicates
the order of which each became prominent. At present there is
concurrent research into all three generations while the first
generation technologies are most highly represented in commercial
production, accounting for 89.6% of 2007 production[6].
[edit] First Generation
First generation cells consist of large-area, high quality and
single junction devices. First Generation technologies involve high
energy and labour inputs which prevent any significant progress in
reducing production costs. Single junction silicon devices are
approaching the theoretical limiting efficiency of 33%[7] and combined with high production costs are unlikely to achieve cost parity with fossil fuel energy generation.
[edit] Second Generation
Second generation materials have been developed to address energy
requirements and production costs of solar cells. Alternative
manufacturing techniques such as vapour deposition and electroplating
are advantageous as they reduce high temperature processing
significantly. It is commonly accepted that as manufacturing techniques
evolve production costs will be dominated by constituent material
requirements,[7] whether this be a silicon substrate, or glass cover. Second generation technologies are expected to gain market share in 2008.[6]
The most successful second generation materials have been cadmium telluride (CdTe), copper indium gallium selenide, amorphous silicon and micromorphous silicon.[6] These materials are applied in a thin film
to a supporting substrate such as glass or ceramics reducing material
mass and therefore costs. These technologies do hold promise of higher
conversion efficiencies, particularly CIGS-CIS, DSC and CdTe
offers significantly cheaper production costs. Among major
manufacturers there is certainly a trend toward second generation
technologies however commercialisation of these technologies has proven
difficult.[8] In 2007 First Solar
produced 200 MW of CdTe solar cells making it the fifth largest
producer of solar cells in 2007 and the first ever to reach the top 10
from production of second generation technologies alone.[8]. Wurth Solar commercialised its CIS technology in 2007 producing 15 MW. Nanosolar commercialised its CIGS technology in 2007 with a production capacity of 430 MW for 2008 in the USA and Germany.[9] In 2007 CdTe production represented 4.7% of total market share, thin film silicon 5.2% and CIGS 0.5%.[8]
[edit] Third Generation
Third generation technologies aim to enhance poor electrical
performance of second generation thin film technologies while
maintaining very low production costs. Current research is targeting
conversion efficiencies of 30-60% while retaining low cost materials
and manufacturing techniques.[7] There are a few approaches to achieving these high efficiencies[10]:
- Multijunction photovoltaic cell.
- Modifying incident spectrum (concentration).
- Use of excess thermal generation to enhance voltages or carrier collection.
[edit] Applications and implementations
Polycrystaline PV cells laminated to backing material in a PV module

Polycrystalline PV cells
Solar cells are often electrically connected and encapsulated as a module. PV modules often have a sheet of glass on the front (sun up) side , allowing light to pass while protecting the semiconductor wafers from the elements (rain, hail, etc.). Solar cells are also usually connected in series in modules, creating an additive voltage.
Connecting cells in parallel will yield a higher current. Modules are
then interconnected, in series or parallel, or both, to create an array with the desired peak DC voltage and current.
The power output of a solar array is measured in watts or kilowatts. In order to calculate the typical energy needs of the application, a measurement in watt-hours, kilowatt-hours or kilowatt-hours per day is often used. A common rule of thumb
is that average power is equal to 20% of peak power, so that each peak
kilowatt of solar array output power corresponds to energy production
of 4.8 kWh per day.
To make practical use of the solar-generated energy, the electricity
is most often fed into the electricity grid using inverters
(grid-connected PV systems); in stand alone systems, batteries are used
to store the energy that is not needed immediately.
[edit] Theory
- See also: Photoelectric effect
[edit] Simple explanation
- Photons in sunlight hit the solar panel and are absorbed by semiconducting materials, such as silicon.
- Electrons (negatively charged) are knocked loose from their atoms, allowing them to flow through the material to produce electricity. The complementary positive charges that are also created (like bubbles) are called holes and flow in the direction opposite of the electrons in a silicon solar panel.
- An array of solar panels converts solar energy into a usable amount of direct current (DC) electricity.
[edit] Photogeneration of charge carriers
When a photon hits a piece of silicon, one of three things can happen:
- the photon can pass straight through the silicon — this (generally) happens for lower energy photons,
- the photon can reflect off the surface,
- the photon can be absorbed by the silicon, if the photon energy is higher than the silicon band gap value. This generates an electron-hole pair and sometimes heat, depending on the band structure.
When a photon is absorbed, its energy is given to an electron in the crystal lattice. Usually this electron is in the valence band,
and is tightly bound in covalent bonds between neighboring atoms, and
hence unable to move far. The energy given to it by the photon
"excites" it into the conduction band,
where it is free to move around within the semiconductor. The covalent
bond that the electron was previously a part of now has one fewer
electron — this is known as a hole. The presence of a missing covalent
bond allows the bonded electrons of neighboring atoms to move into the
"hole," leaving another hole behind, and in this way a hole can move
through the lattice. Thus, it can be said that photons absorbed in the
semiconductor create mobile electron-hole pairs.
A photon need only have greater energy than that of the band gap in
order to excite an electron from the valence band into the conduction
band. However, the solar frequency spectrum approximates a black body spectrum at ~6000 K, and as such, much of the solar radiation reaching the Earth
is composed of photons with energies greater than the band gap of
silicon. These higher energy photons will be absorbed by the solar
cell, but the difference in energy between these photons and the
silicon band gap is converted into heat (via lattice vibrations —
called phonons) rather than into usable electrical energy.
[edit] Charge carrier separation
There are two main modes for charge carrier separation in a solar cell:
- drift of carriers, driven by an electrostatic field established across the device
- diffusion of carriers from zones of high carrier
concentration to zones of low carrier concentration (following a
gradient of electrochemical potential).
In the widely used p-n junction solar cells, the dominant mode of
charge carrier separation is by drift. However, in non-p-n-junction
solar cells (typical of the third generation of solar cell research
such as dye and polymer thin-film solar cells), a general electrostatic
field has been confirmed to be absent, and the dominant mode of
separation is via charge carrier diffusion.[11]
[edit] The p-n junction
The most commonly known solar cell is configured as a large-area p-n junction
made from silicon. As a simplification, one can imagine bringing a
layer of n-type silicon into direct contact with a layer of p-type
silicon. In practice, p-n junctions of silicon solar cells are not made
in this way, but rather, by diffusing an n-type dopant into one side of
a p-type wafer (or vice versa).
If a piece of p-type silicon is placed in intimate contact with a piece of n-type silicon, then a diffusion
of electrons occurs from the region of high electron concentration (the
n-type side of the junction) into the region of low electron
concentration (p-type side of the junction). When the electrons diffuse
across the p-n junction, they recombine with holes on the p-type side.
The diffusion of carriers does not happen indefinitely however, because
of an electric field
which is created by the imbalance of charge immediately on either side
of the junction which this diffusion creates. The electric field
established across the p-n junction creates a diode that promotes current
to flow in only one direction across the junction. Electrons may pass
from the n-type side into the p-type side, and holes may pass from the
p-type side to the n-type side, but not the other way around.[12] This region where electrons have diffused across the junction is called the depletion region because it no longer contains any mobile charge carriers. It is also known as the "space charge region".
[edit] Connection to an external load
Ohmic metal-semiconductor
contacts are made to both the n-type and p-type sides of the solar
cell, and the electrodes connected to an external load. Electrons that
are created on the n-type side, or have been "collected" by the
junction and swept onto the n-type side, may travel through the wire,
power the load, and continue through the wire until they reach the
p-type semiconductor-metal contact. Here, they recombine with a hole
that was either created as an electron-hole pair on the p-type side of
the solar cell, or swept across the junction from the n-type side after
being created there.
[edit] Equivalent circuit of a solar cell
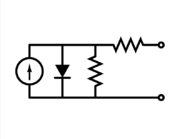
The equivalent circuit of a solar cell
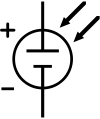
The schematic symbol of a solar cell
To understand the electronic behavior of a solar cell, it is useful to create a model
which is electrically equivalent, and is based on discrete electrical
components whose behavior is well known. An ideal solar cell may be
modelled by a current source in parallel with a diode; in practice no solar cell is ideal, so a shunt resistance and a series resistance component are added to the model.[13]
The resulting equivalent circuit of a solar cell is shown on the left.
Also shown, on the right, is the schematic representation of a solar
cell for use in circuit diagrams.
[edit] Circuit Equations defining solar cell
The equations which describe the I-V characteristics of the cell are
|
[edit] Solar cell efficiency factors
[edit] Sun unit
The irradiance of the sun on the outer atmosphere when the sun and
earth are spaced at 1 AU—the mean earth/sun distance of 149,597,870
km—is called the solar constant. Currently accepted values are about
1360 W/m² (the NASA value given in ASTM E 490-73a is 1353 ±21 W/m²).
The World Metrological Organization (WMO) promotes a value of 1367
W/m². The solar constant is the total integrated irradiance over the
entire spectrum (the area under the curve in Fig. 1 plus the 3.7% at
shorter and longer wavelengths. The irradiance falling on the earth's
atmosphere changes over a year by about 6.6% due to the variation in
the earth/sun distance. Solar activity variations cause irradiance
changes of up to 1%. For a solar simulator, it is convenient to
describe the irradiance of the simulator in “suns.” One “sun” is
equivalent to irradiance of one solar constant.[citation needed]
[edit] Energy conversion efficiency
A solar cell's energy conversion efficiency (η,
"eta"), is the percentage of power converted (from absorbed light to
electrical energy) and collected, when a solar cell is connected to an
electrical circuit. This term is calculated using the ratio of the
maximum power point, Pm, divided by the input light irradiance (E, in W/m²) under standard test conditions (STC) and the surface area of the solar cell (Ac in m²).
STC specifies a temperature of 25°C and an irradiance of 1000 W/m²
with an air mass 1.5 (AM1.5) spectrum. These correspond to the
irradiance and spectrum of sunlight incident on a clear day upon a
sun-facing 37°-tilted surface with the sun at an angle of 41.81° above
the horizon.[14][15]
This condition approximately represents solar noon near the spring and
autumn equinoxes in the continental United States with surface of the
cell aimed directly at the sun. Thus, under these conditions a solar
cell of 12% efficiency with a 100 cm2 (0.01 m2) surface area can be expected to produce approximately 1.2 watts of power.
The losses of a solar cell may be broken down into reflectance
losses, thermodynamic efficiency, recombination losses and resistive
electrical loss. The overall efficiency is the product of each of these
individual losses.
Due to the difficulty in measuring these parameters directly, other
parameters are measured instead: Thermodynamic Efficiency, Quantum
Efficiency, VOC ratio, and Fill Factor. Reflectance losses
are a portion of the Quantum Efficiency under "External Quantum
Efficiency". Recombination losses make up a portion of the Quantum
Efficiency, VOC ratio, and Fill Factor. Resistive losses are
predominantly categorized under Fill Factor, but also make up minor
portions of the Quantum Efficiency, VOC ratio.
[edit] Thermodynamic Efficiency Limit
Solar cells operate as quantum energy conversion devices, and are
therefore subject to the "Thermodynamic Efficiency Limit". Photons with
an energy below the band gap of the absorber material cannot generate a
hole-electron pair, and so their energy is not converted to useful
output and only generates heat if absorbed. For photons with an energy
above the band gap energy, only a fraction of the energy above the band
gap can be converted to useful output. When a photon of greater energy
is absorbed, the excess energy above the band gap is converted to
kinetic energy of the carrier combination. The excess kinetic energy is
converted to heat through phonon interactions as the kinetic energy of
the carriers slows to equilibrium velocity.
Solar cells with multiple band gap absorber materials are able to
more efficiently convert the solar spectrum. By using multiple band
gaps, the solar spectrum may be broken down into smaller bins where the
thermodynamic efficiency limit is higher for each bin.[16]
[edit] Quantum efficiency
As described above, when a photon is absorbed by a solar cell it is
converted to an electron-hole pair. This electron-hole pair may then
travel to the surface of the solar cell and contribute to the current
produced by the cell; such a carrier is said to be collected.
Alternatively, the carrier may give up its energy and once again become
bound to an atom within the solar cell without reaching the surface;
this is called recombination, and carriers that recombine do not contribute to the production of electrical current.
Quantum efficiency
refers to the percentage of photons that are converted to electric
current (i.e., collected carriers) when the cell is operated under
short circuit conditions. External quantum efficiency is the fraction of incident photons that are converted to electrical current, while internal quantum efficiency is the fraction of absorbed
photons that are converted to electrical current. Mathematically,
internal quantum efficiency is related to external quantum efficiency
by the reflectance of the solar cell; given a perfect anti-reflection
coating, they are the same.
Quantum efficiency should not be confused with energy conversion efficiency,
as it does not convey information about the power collected from the
solar cell. Furthermore, quantum efficiency is most usefully expressed
as a spectral measurement (that is, as a function of photon
wavelength or energy). Since some wavelengths are absorbed more
effectively than others in most semiconductors, spectral measurements
of quantum efficiency can yield information about which parts of a
particular solar cell design are most in need of improvement.
[edit] VOC ratio
Due to recombination, the open circuit voltage (VOC) of
the cell will be below the band gap voltage of the cell. Since the
energy of the photons must be at or above the band gap to generate a
carrier pair, cell voltage below the band gap voltage represents a
loss. This loss is represented by the ratio of VOC divided by VG
[edit] Maximum-power point
A solar cell may operate over a wide range of voltages (V) and currents (I). By increasing the resistive load on an irradiated cell continuously from zero (a short circuit) to a very high value (an open circuit) one can determine the maximum-power
point, the point that maximizes V×I; that is, the load for which the
cell can deliver maximum electrical power at that level of irradiation.
(The output power is zero in both the short circuit and open circuit
extremes).
A high quality, monocrystalline silicon solar cell, at 25 °C cell
temperature, may produce 0.60 volts open-circuit (Voc). The cell
temperature in full sunlight, even with 25 °C air temperature, will
probably be close to 45 °C, reducing the open-circuit voltage to 0.55
volts per cell. The voltage drops modestly, with this type of cell,
until the short-circuit current is approached (Isc). Maximum power
(with 45 °C cell temperature) is typically produced with 75% to 80% of
the open-circuit voltage (0.43 volts in this case) and 90% of the
short-circuit current. This output can be up to 70% of the Voc x Isc
product. The short-circuit current (Isc) from a cell is nearly
proportional to the illumination, while the open-circuit voltage (Voc)
may drop only 10% with a 80% drop in illumination. Lower-quality cells
have a more rapid drop in voltage with increasing current and could
produce only 1/2 Voc at 1/2 Isc. The usable power output could thus
drop from 70% of the Voc x Isc product to 50% or even as little as 25%.
Vendors who rate their solar cell "power" only as Voc x Isc, without
giving load curves, can be seriously distorting their actual
performance.
The maximum power point of a photovoltaic varies with incident illumination. For systems large enough to justify the extra expense, a maximum power point tracker tracks the instantaneous power by continually measuring the voltage and current (and hence, power transfer), and uses this information to dynamically adjust the load so the maximum power is always transferred, regardless of the variation in lighting.
[edit] Fill factor
Another defining term in the overall behavior of a solar cell is the fill factor (FF). This is the ratio of the maximum power point divided by the open circuit voltage (Voc) and the short circuit current (Isc):
[edit] Comparison of energy conversion efficiencies
At this point, discussion of the different ways to calculate
efficiency for space cells and terrestrial cells is necessary to
alleviate confusion. In space, where there is no atmosphere, the
spectrum of the sun is relatively unfiltered. However on earth, with
air filtering the incoming light, the solar spectrum changes. To
account for the spectral differences, a system was devised to calculate
this filtering effect. Simply, the filtering effect ranges from Air Mass
0 (AM0) in space, to approximately Air Mass 1.5 on earth. Multiplying
the spectral differences by the quantum efficiency of the solar cell in
question will yield the efficiency of the device. For example, a
Silicon solar cell in space might have an efficiency of 14% at AM0, but
have an efficiency of 16% on earth at AM 1.5. Terrestrial efficiencies
typically are greater than space efficiencies.
Solar cell efficiencies vary from 6% for amorphous silicon-based
solar cells to 40.7% with multiple-junction research lab cells and
42.8% with multiple dies assembled into a hybrid package.[17] Solar cell energy conversion efficiencies for commercially available multicrystalline Si solar cells are around 14-19%[18].
The highest efficiency cells have not always been the most economical —
for example a 30% efficient multijunction cell based on exotic
materials such as gallium arsenide or indium selenide and produced in
low volume might well cost one hundred times as much as an 8% efficient
amorphous silicon cell in mass production, while only delivering about
four times the electrical power.
However, there is a way to "boost" solar power. By increasing the
light intensity, typically photogenerated carriers are increased,
resulting in increased efficiency by up to 15%. These so-called
"concentrator systems" have only begun to become cost-competitive as a
result of the development of high efficiency GaAs cells. The increase
in intensity is typically accomplished by using concentrating optics. A
typical concentrator system may use a light intensity 6-400 times the
sun, and increase the efficiency of a one sun GaAs cell from 31% at AM
1.5 to 35%.
A common method used to express economic costs of electricity-generating systems is to calculate a price per delivered kilowatt-hour
(kWh). The solar cell efficiency in combination with the available
irradiation has a major influence on the costs, but generally speaking
the overall system efficiency is important. Using the commercially
available solar cells (as of 2006) and system technology leads to
system efficiencies between 5 and 19%. As of 2005, photovoltaic
electricity generation costs ranged from ~0.60 US$/kWh (0.50 €/kWh)
(central Europe) down to ~0.30 US$/kWh (0.25 €/kWh) in regions of high
solar irradiation. This electricity is generally fed into the
electrical grid on the customer's side of the meter. The cost can be
compared to prevailing retail electric pricing (as of 2005), which
varied from between 0.04 and 0.50 US$/kWh worldwide. (Note: in addition
to solar irradiance profiles, these costs/kwh calculations will vary
depending on assumptions for years of useful life of a system. Most
c-Si panels are warrantied for 25 years and should see 35+ years of
useful life.)
The chart at the right illustrates the various commercial large-area
module energy conversion efficiencies and the best laboratory
efficiencies obtained for various materials and technologies.

Reported timeline of solar cell energy conversion efficiencies (from National Renewable Energy Laboratory (USA)
[edit] Watts peak
Since solar cell output power depends on multiple factors, such as the sun's incidence angle,
for comparison purposes between different cells and panels, the measure
of watts peak (Wp) is used. It is the output power under these
conditions known as STC:[19][20]
- insolation (solar irradiance) 1000 W/m²
- solar reference spectrum AM (airmass) 1.5
- cell temperature 25°C
[edit] Solar cells and energy payback
In the 1990s, when silicon cells were twice as thick, efficiencies
were 30% lower than today and lifetimes were shorter, it may well have
cost more energy to make a cell than it could generate in a lifetime.
In the meantime, the technology has progressed significantly, and the
energy payback time of a modern photovoltaic module is typically from 1
to 4 years [21] depending on the type and where it is used (see net energy gain).
With a typical lifetime of 20 to 30 years, this means that modern solar
cells are net energy producers, i.e they generate much more energy over
their lifetime than the energy expended in producing them.[22][21][23]
[edit] Light-absorbing materials
All solar cells require a light absorbing material contained within the cell structure to absorb photons and generate electrons via the photovoltaic effect.
The materials used in solar cells tend to have the property of
preferentially absorbing the wavelengths of solar light that reach the
earth surface; however, some solar cells are optimized for light
absorption beyond Earth's atmosphere as well. Light absorbing materials
can often be used in multiple physical configurations to take
advantage of different light absorption and charge separation
mechanisms. Many currently available solar cells are configured as bulk
materials that are subsequently cut into wafers and treated in a
"top-down" method of synthesis (silicon being the most prevalent bulk
material). Other materials are configured as thin-films (inorganic layers, organic dyes, and organic polymers) that are deposited on supporting substrates, while a third group are configured as nanocrystals and used as quantum dots
(electron-confined nanoparticles) embedded in a supporting matrix in a
"bottom-up" approach. Silicon remains the only material that is
well-researched in both bulk and thin-film configurations.
There are many new alternatives to Silicon photocells. Proprietary
nano-particle silicon printing processes promises many of the
photovoltaic features that conventional silicon can never achieve. It
can be printed reel to reel on stainless steel or other high
temperature substrates [24].
However, most of the work on the next generation of photovoltaics is
directed at printing onto low cost flexible polymer film and ultimately
on common packaging materials. The main contenders are currently CIGS, CdTe, DSSC and organic photovoltaics[24] .
The following is a current list of light absorbing materials, listed by configuration and substance-name:
[edit] Bulk
These bulk technologies are often referred to as wafer-based
manufacturing. In other words, in each of these approaches,
self-supporting wafers between 180 to 240 micrometers thick are
processed and then soldered together to form a solar cell module. A
general description of silicon wafer processing is provided in Manufacture and Devices.
[edit] Silicon
Basic structure of a silicon based solar cell and its working mechanism.
By far, the most prevalent bulk material for solar cells is crystalline silicon (abbreviated as a group as c-Si),
also known as "solar grade silicon". Bulk silicon is separated into
multiple categories according to crystallinity and crystal size in the
resulting ingot, ribbon, or wafer.
- monocrystalline silicon (c-Si): often made using the Czochralski process.
Single-crystal wafer cells tend to be expensive, and because they are
cut from cylindrical ingots, do not completely cover a square solar
cell module without a substantial waste of refined silicon. Hence most c-Si panels have uncovered gaps at the four corners of the cells. - Poly- or multicrystalline silicon (poly-Si or mc-Si): made
from cast square ingots — large blocks of molten silicon carefully
cooled and solidified. These cells are less expensive to produce than
single crystal cells but are less efficient. - Ribbon silicon: formed by drawing flat thin films from
molten silicon and having a multicrystalline structure. These cells
have lower efficiencies than poly-Si, but save on production costs due
to a great reduction in silicon waste, as this approach does not
require sawing from ingots.
[edit] Thin films
The various thin-film technologies currently being developed reduce the amount (or mass) of light absorbing material required in creating a solar cell.
This can lead to reduced processing costs from that of bulk materials
(in the case of silicon thin films) but also tends to reduce energy conversion efficiency, although many multi-layer thin films have efficiencies above those of bulk silicon wafers.
[edit] CdTe
Cadmium telluride
is an efficient light-absorbing material for thin-film solar cells.
Compared to other thin-film materials, CdTe is easier to deposit and
more suitable for large-scale production. Despite much discussion of
the toxicity of CdTe-based solar cells, this is the only technology
(apart from amorphous silicon) that can be delivered on a large scale[citation needed]. The perception of the toxicity of CdTe is based on the toxicity of elemental cadmium, a heavy metal that is a cumulative poison.
However it has been shown that the release of cadmium to the atmosphere
is lower with CdTe-based solar cells than with silicon photovoltaics
and other thin-film solar cell technologies. [25]
[edit] Copper-Indium Selenide
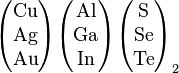 Possible combinations of I III VI elements in the periodic table that have photovoltaic effect |
The materials based on CuInSe2 that are of interest for
photovoltaic applications include several elements from groups I, III
and VI in the periodic table. These semiconductors are especially
attractive for thin film solar cell application because of their high
optical absorption coefficients and versatile optical and electrical
characteristics which can in principle be manipulated and tuned for a
specific need in a given device. CIS is an abbreviation for general
chalcopyrite films of copper indium selenide (CuInSe2), CIGS mentioned below is a variation of CIS. CIS films (no Ga) achieved greater than 14% efficiency.[26]
However, manufacturing costs of CIS solar cells at present are high
when compared with amorphous silicon solar cells but continuing work is
leading to more cost-effective production processes. The first
large-scale production of CIS modules was started in 2006 in Germany by
Wuerth Solar.
When gallium is substituted for some of the indium in CIS, the material is sometimes called CIGS , or copper indium/gallium diselenide, a solid mixture of the semiconductors CuInSe2 and CuGaSe2, often abbreviated by the chemical formula CuInxGa(1-x)Se2. Unlike the conventional silicon based solar cell, which can be modelled as a simple p-n junction (see under semiconductor),
these cells are best described by a more complex heterojunction model.
The best efficiency of a thin-film solar cell as of March 2008 was
19.9% with CIGS absorber layer.[27]
Higher efficiencies (around 30%) can be obtained by using optics to
concentrate the incident light. The use of gallium increases the
optical bandgap of the CIGS layer as compared to pure CIS, thus
increasing the open-circuit voltage. In another point of view, gallium
is added to replace as much indium as possible due to gallium's
relative availability to indium. Approximately 70%[28]
of indium currently produced is used by the flat-screen monitor
industry. Some investors in solar technology worry that production of
CIGS cells will be limited by the availability of indium. Producing 2
GW of CIGS cells (roughly the amount of silicon cells produced in 2006)
would use about 10% of the indium produced in 2004.[29] For comparison, silicon solar cells used up 33% of the world's electronic grade silicon production in 2006. Nanosolar
claims to waste only 5% of the indium it uses. As of 2006, the best
conversion efficiency for flexible CIGS cells on polyimide is 14.1% by
Tiwari et al, at the ETH, Switzerland.
That being said, indium can easily be recycled from decommissioned
PV modules. The recycling program in Germany is an example that
highlights the regenerative industrial paradigm: "From cradle to cradle".
Selenium allows for better uniformity across the layer and so the
number of recombination sites in the film are reduced which benefits
the quantum efficiency and thus the conversion efficiency.[citation needed]
[edit] Gallium arsenide (GaAs) multijunction
High-efficiency multijunction cells were originally developed for special applications such as satellites and space exploration, but at present, their use in terrestrial concentrators might be the lowest cost alternative in terms of $/kWh and $/W.[30] These multijunction cells consist of multiple thin films produced using Metalorganic vapour phase epitaxy. A triple-junction cell, for example, may consist of the semiconductors: GaAs, Ge, and GaInP2.[31] Each type of semiconductor will have a characteristic band gap energy which, loosely speaking, causes it to absorb light most efficiently at a certain color, or more precisely, to absorb electromagnetic radiation
over a portion of the spectrum. The semiconductors are carefully chosen
to absorb nearly all of the solar spectrum, thus generating electricity
from as much of the solar energy as possible.
GaAs based multijunction devices are the most efficient solar cells
to date, reaching a record high of 40.7% efficiency under solar
concentration and laboratory conditions.[32]
This technology is currently being utilized in the Mars rover missions.
Tandem solar cells based on monolithic, series connected, gallium
indium phosphide (GaInP), gallium arsenide GaAs, and germanium Ge pn
junctions, are seeing demand rapidly rise. In just the past 12 months
(12/2006 - 12/2007), the cost of 4N gallium metal has risen from about
$350 per kg to $680 per kg. Additionally, germanium metal prices have
risen substantially to $1000-$1200 per kg this year. Those materials
include gallium (4N, 6N and 7N Ga), arsenic (4N, 6N and 7N) and
germanium, pyrolitic boron nitride (pBN) crucibles for growing
crystals, and boron oxide, these products are critical to the entire
substrate manufacturing industry.
Triple-junction GaAs solar cells were also being used as the power source of the Dutch four-time World Solar Challenge winners Nuna in 2005 and 2007, and also by the Dutch solar cars Solutra (2005) and Twente One (2007).
The dutch Radboud University Nijmegen set the record for thin film solar cell effiency using a single junction GaAs to 25.8% in august 2008 using only 4 µm thick GaAs layer which can be transferred from a wafer base to glas or plastic film. [33]
[edit] Light-absorbing dyes (DSSC)
Typically a ruthenium metalorganic dye (Ru-centered) is used as a
monolayer of light-absorbing material. The dye-sensitized solar cell
depends on a mesoporous layer of nanoparticulate titanium dioxide to greatly amplify the surface area (200-300 m²/g TiO2, as compared to approximately 10 m²/g of flat single crystal). The photogenerated electrons from the light absorbing dye are passed on to the n-type TiO2,
and the holes are passed to an electrolyte on the other side of the
dye. The circuit is completed by a redox couple in the electrolyte,
which can be liquid or solid. This type of cell allows a more flexible
use of materials, and is typically manufactured by screen printing,
with the potential for lower processing costs than those used for bulk
solar cells. However, the dyes in these cells also suffer from
degradation under heat and UV light, and the cell casing is difficult
to seal due to the solvents used in assembly. In spite of the above,
this is a popular emerging technology with some commercial impact
forecast within this decade.
[edit] Organic/polymer solar cells
Organic solar cells and Polymer solar cells are built from thin films (typically 100 nm) of organic semiconductors such as polymers and small-molecule compounds like polyphenylene vinylene, copper phthalocyanine (a blue or green organic pigment) and carbon fullerenes.
Energy conversion efficiencies achieved to date using conductive
polymers are low compared to inorganic materials, with the highest
reported efficiency of 6.5% [34]
for a tandem cell architecture. However, these cells could be
beneficial for some applications where mechanical flexibility and
disposability are important.
These devices differ from inorganic semiconductor solar cells in
that they do not rely on the large built in electric field of a PN
junction to separate the electrons and holes created when photons are
absorbed. The active region of an organic device consists of two
materials, one which acts as an electron donor and the other as an
acceptor. When a photon is converted into an electron hole pair,
typically in the donor material, the charges tend to remain bound in
the form of an exciton,
and are separated when the exciton diffuses to the donor-acceptor
interface. The short exciton diffusion lengths of most polymer systems
tend to limit the efficiency of such devices. Nanostructured
interfaces, sometimes in the form of bulk heterojunctions, can improve
performance [35].
[edit] Silicon Thin Films
Silicon thin-films are mainly deposited by chemical vapor deposition (typically plasma-enhanced (PE-CVD)) from silane gas and hydrogen gas. Depending on the deposition's parameters, this can yield:
- Amorphous silicon (a-Si or a-Si:H)
- protocrystalline silicon or
- Nanocrystalline silicon (nc-Si or nc-Si:H).
These types of silicon present dangling and twisted bonds, which
results in deep defects (energy levels in the bandgap) as well as
deformation of the valence and conduction bands (band tails). The solar
cells made from these materials tend to have lower energy conversion efficiency than bulk silicon, but are also less expensive to produce. The quantum efficiency of thin film solar cells is also lower due to reduced number of collected charge carriers per incident photon.
Amorphous silicon has a higher bandgap (1.7 eV) than crystalline
silicon (c-Si) (1.1 eV), which means it absorbs the visible part of the
solar spectrum more strongly than the infrared portion of the spectrum. As nc-Si has about the same bandgap as c-Si, the two material can be combined in thin layers, creating a layered cell called a tandem cell.
The top cell in a-Si absorbs the visible light and leaves the infrared
part of the spectrum for the bottom cell in nanocrystalline Si.
Recently, solutions to overcome the limitations of thin-film
crystalline silicon have been developed. Light trapping schemes where
the incoming light is obliquely coupled into the silicon and the light
traverses the film several times enhance the absorption of sunlight in
the films. Thermal processing techniques enhance the crystallinity of
the silicon and pacify electronic defects.[citation needed]
A silicon thin film technology is being developed for building
integrated photovoltaics (BIPV) in the form of semi-transparent solar
cells which can be applied as window glazing. These cells function as
window tinting while generating electricity.
[edit] Nanocrystalline solar cells
These structures make use of some of the same thin-film light
absorbing materials but are overlain as an extremely thin absorber on a
supporting matrix of conductive polymer or mesoporous metal oxide
having a very high surface area to increase internal reflections (and
hence increase the probability of light absorption). Using nanocrystals
allows one to design architectures on the length scale of nanometers,
the typical exciton diffusion length. In particular, single-nanocrystal
('channel') devices, an array of single p-n junctions between the
electrodes and separated by a period of about a diffusion length,
represent a new architecture for solar cells and potentially high
efficiency.
[edit] Concentrating photovoltaics (CPV)
Concentrating photovoltaic systems use a large area of lenses or mirrors to focus sunlight on a small area of photovoltaic cells.[36] If these systems use single or dual-axis tracking to improve performance, they may be referred to as Heliostat Concentrator Photovoltaics
(HCPV). The primary attraction of CPV systems is their reduced usage of
semiconducting material which is expensive and currently in short
supply. Additionally, increasing the concentration ratio improves the
performance of general photovoltaic materials.[37]
Despite the advantages of CPV technologies their application has been
limited by the costs of focusing, tracking and cooling equipment. On October 25, 2006, the Australian federal government and the Victorian state government together with photovoltaic technology company Solar Systems announced a project using this technology, Solar power station in Victoria,
planned to come online in 2008 and be completed by 2013. This plant, at
154 MW, would be ten times larger than the largest current photovoltaic
plant in the world.[38]
[edit] Silicon solar cell device manufacture

Solar powered scientific calculator
Because solar cells are semiconductor devices, they share many of
the same processing and manufacturing techniques as other semiconductor
devices such as computer and memory chips.
However, the stringent requirements for cleanliness and quality control
of semiconductor fabrication are a little more relaxed for solar cells.
Most large-scale commercial solar cell factories today make screen
printed poly-crystalline silicon solar cells. Single crystalline wafers
which are used in the semiconductor industry can be made into excellent
high efficiency solar cells, but they are generally considered to be
too expensive for large-scale mass production.
Poly-crystalline silicon wafers are made by wire-sawing block-cast
silicon ingots into very thin (180 to 350 micrometer) slices or wafers.
The wafers are usually lightly p-type doped. To make a solar cell from the wafer, a surface diffusion of n-type dopants is performed on the front side of the wafer. This forms a p-n junction a few hundred nanometers below the surface.
Antireflection coatings, which increase the amount of light coupled
into the solar cell, are typically next applied. Over the past decade,
silicon nitride has gradually replaced titanium dioxide as the
antireflection coating of choice because of its excellent surface
passivation qualities (i.e., it prevents carrier recombination at the
surface of the solar cell). It is typically applied in a layer several
hundred nanometers thick using plasma-enhanced chemical vapor
deposition (PECVD). Some solar cells have textured front surfaces that,
like antireflection coatings, serve to increase the amount of light
coupled into the cell. Such surfaces can usually only be formed on
single-crystal silicon, though in recent years methods of forming them
on multicrystalline silicon have been developed.
The wafer then has a full area metal contact made on the back
surface, and a grid-like metal contact made up of fine "fingers" and
larger "busbars" are screen-printed onto the front surface using a silver
paste. The rear contact is also formed by screen-printing a metal
paste, typically aluminium. Usually this contact covers the entire rear
side of the cell, though in some cell designs it is printed in a grid
pattern. The paste is then fired at several hundred degrees Celsius to
form metal electrodes in ohmic contact
with the silicon. After the metal contacts are made, the solar cells
are interconnected in series (and/or parallel) by flat wires or metal
ribbons, and assembled into modules or "solar panels". Solar panels have a sheet of tempered glass on the front, and a polymer
encapsulation on the back. Tempered glass cannot be used with amorphous
silicon cells because of the high temperatures during the deposition
process.
[edit] Lifespan
A solar cell must be capable of producing electricity for at least twenty years, without a significant decrease in efficiency.
[edit] Low cost solar cells
Dye-sensitized solar cell is considered the low cost solar cell.
This cell is extremely promising because it is made of low-cost
materials and does not need elaborate apparatus to manufacture, so it
can be made in a DIY
way allowing more players to produce it than any other type of solar
cell. In bulk it should be significantly less expensive than older solid-state cell designs. It can be engineered into flexible sheets. Although its conversion efficiency is less than the best thin film cells, its price/performance ratio should be high enough to allow them to compete with fossil fuel electrical generation.
[edit] Current research on materials and devices
- See also: Timeline of solar cells
There are currently many research groups active in the field of photovoltaics in universities
and research institutions around the world. This research can be
divided into three areas: making current technology solar cells cheaper
and/or more efficient to effectively compete with other energy sources;
developing new technologies based on new solar cell architectural
designs; and developing new materials to serve as light absorbers and
charge carriers.
[edit] Silicon processing
One way of reducing the cost is to develop cheaper methods of
obtaining silicon that is sufficiently pure. Silicon is a very common
element, but is normally bound in silica, or silica sand. Processing silica (SiO2)
to produce silicon is a very high energy process - at current
efficiencies, it takes over two years for a conventional solar cell to
generate as much energy as was used to make the silicon it contains.[39]
More energy efficient methods of synthesis are not only beneficial to
the solar industry, but also to industries surrounding silicon
technology as a whole.
The current industrial production of silicon is via the reaction
between carbon (charcoal) and silica at a temperature around 1700 degrees Celsius.
In this process, known as carbothermic reduction, each tonne of silicon
(metallurgical grade, about 98% pure) is produced with the emission of
about 1.5 tonnes of carbon dioxide.
Solid silica can be directly converted (reduced) to pure silicon by
electrolysis in a molten salt bath at a fairly mild temperature (800 to
900 degrees Celsius).[40][41] While this new process is in principle the same as the FFC Cambridge Process
which was first discovered in late 1996, the interesting laboratory
finding is that such electrolytic silicon is in the form of porous
silicon which turns readily into a fine powder, (with a particle size
of a few micrometres), and may therefore offer new opportunities for
development of solar cell technologies.
Another approach is also to reduce the amount of silicon used and
thus cost, is by micromachining wafers into very thin, virtually
transparent layers that could be used as transparent architectural
coverings.[42]
. The technique involves taking a silicon wafer, typically 1 to 2 mm
thick, and making a multitude of parallel, transverse slices across the
wafer, creating a large number of slivers that have a thickness of 50
micrometres and a width equal to the thickness of the original wafer.
These slices are rotated 90 degrees, so that the surfaces corresponding
to the faces of the original wafer become the edges of the slivers. The
result is to convert, for example, a 150 mm diameter, 2 mm-thick wafer
having an exposed silicon surface area of about 175 cm² per side into
about 1000 slivers having dimensions of 100 mm x 2 mm x 0.1 mm,
yielding a total exposed silicon surface area of about 2000 cm² per
side. As a result of this rotation, the electrical doping and contacts
that were on the face of the wafer are located the edges of the sliver,
rather than the front and rear as is the case with conventional wafer
cells. This has the interesting effect of making the cell sensitive
from both the front and rear of the cell (a property known as
bifaciality).[43]
Using this technique, one silicon wafer is enough to build a 140 watt
panel, compared to about 60 wafers needed for conventional modules of
same power output.
[edit] Thin-film processing
Thin-film solar cells use less than 1% of the raw material (silicon
or other light absorbers) compared to wafer based solar cells, leading
to a significant price drop per kWh. There are many research groups
around the world actively researching different thin-film approaches
and/or materials, however it remains to be seen[vague] if these solutions can generate the same space-efficiency as traditional silicon processing.
One particularly promising technology is crystalline silicon thin
films on glass substrates. This technology makes use of the advantages
of crystalline silicon as a solar cell material, with the cost savings
of using a thin-film approach.[citation needed]
Another interesting aspect of thin-film solar cells is the
possibility to deposit the cells on all kind of materials, including
flexible substrates (PET for example), which opens a new dimension for new applications.[citation needed]
One of the R&D Magazine's prestigious R&D 100 Awards — also called the “Oscars of Invention”— for 2008, has gone to National Renewable Energy Laboratory Hybrid CGIS
(or Thin-Film Photovoltaic Manufacturing Process) – hybrid CIGS cells
that are manufactured in layers by using ink-jet and ultrasonic
technology to precisely apply metal-organic inks in separate layers
directly into common building materials such as metal and glass [44].
[edit] Metamorphic Multijunction Solar Cell
The National Renewable Energy Laboratory won another R&D Magazine's R&D 100 Awards
for its Metamorphic Multijunction Solar Cell, an ultra-light and
flexible cell that converts solar energy with record efficiency [44].
The ultra-light, highly efficient solar cell was developed at NREL and is being commercialized by Emcore Corp. [45] of Albuquerque, N.M., in partnership with the Air Force Research Laboratories Space Vehicles Directorate at Kirtland Air Force Base in Albuquerque.
It represents a new class of solar cells with clear advantages in
performance, engineering design, operation and cost. For decades,
conventional cells have featured wafers of semiconducting materials with similar crystalline
structure. Their performance and cost effectiveness is constrained by
growing the cells in an upright configuration. Meanwhile, the cells are
rigid, heavy and thick with a bottom layer made of germanium.
In the new method, the cell is grown upside down. These layers use
high-energy materials with extremely high quality crystals, especially
in the upper layers of the cell where most of the power is produced.
Not all of the layers follow the lattice
pattern of even atomic spacing. Instead, the cell includes a full range
of atomic spacing, which allows for greater absorption and use of
sunlight. The thick, rigid germanium layer
is removed, reducing the cell's cost and 94% of its weight. By turning
the conventional approach to cells on its head, the result is an
ultra-light and flexible cell that also converts solar energy with
record efficiency (40.8 percent under 326 suns concentration).
[edit] Polymer processing
The invention of conductive polymers (for which Alan Heeger, Alan G. MacDiarmid and Hideki Shirakawa were awarded a Nobel prize) may lead to the development of much cheaper cells that are based on inexpensive plastics. However, all organic solar cells made to date suffer from degradation upon exposure to UV light, and hence have lifetimes which are far too short to be viable. The conjugated
double bond systems in the polymers, which carry the charge, are always
susceptible to breaking up when radiated with shorter wavelengths.
Additionally, most conductive polymers, being highly unsaturated and
reactive, are highly sensitive to atmospheric moisture and oxidation,
making commercial applications difficult.
[edit] Nanoparticle processing
Experimental non-silicon solar panels can be made of quantum heterostructures, eg. carbon nanotubes or quantum dots, embedded in conductive polymers
or mesoporous metal oxides. In addition, thin films of many of these
materials on conventional silicon solar cells can increase the optical
coupling efficiency into the silicon cell, thus boosting the overall
efficiency. By varying the size of the quantum dots, the cells can be
tuned to absorb different wavelengths. Although the research is still
in its infancy, quantum dot-modified
photovoltaics may be able to achieve up to 42 percent energy conversion
efficiency due to multiple exciton generation(MEG).[46]
Researchers at the University of California, San Diego have come up with a way of making solar photovoltaic cells more efficient by making them fuzzy with indium phosphide nanowires. It sounds similar to a project announced by a consortium of German universities, working in concert with Harvard University Science department.[47]
[edit] Transparent conductors
Many new solar cells use transparent thin films that are also
conductors of electrical charge. The dominant conductive thin films
used in research now are transparent conductive oxides (abbreviated
"TCO"), and include fluorine-doped tin oxide (SnO2:F, or "FTO"), doped zinc oxide (e.g.: ZnO:Al), and indium tin oxide
(abbreviated "ITO"). These conductive films are also used in the LCD
industry for flat panel displays. The dual function of a TCO allows
light to pass through a substrate window to the active light absorbing
material beneath, and also serves as an ohmic contact to transport
photogenerated charge carriers away from that light absorbing material.
The present TCO materials are effective for research, but perhaps are
not yet optimized for large-scale photovoltaic production. They require
very special deposition conditions at high vacuum, they can sometimes
suffer from poor mechanical strength, and most have poor transmittance
in the infrared portion of the spectrum (e.g.: ITO thin films can also
be used as infrared filters in airplane windows). These factors make
large-scale manufacturing more costly.
A relatively new area has emerged using carbon nanotube networks as a transparent conductor for organic solar cells.
Nanotube networks are flexible and can be deposited on surfaces a
variety of ways. With some treatment, nanotube films can be highly
transparent in the infrared, possibly enabling efficient low bandgap
solar cells. Nanotube networks are p-type conductors, whereas
traditional transparent conductors are exclusively n-type. The availability of a p-type transparent conductor could lead to new cell designs that simplify manufacturing and improve efficiency.
[edit] Silicon wafer based solar cells
Despite the numerous attempts at making better solar cells by using
new and exotic materials, the reality is that the photovoltaics market
is still dominated by silicon wafer-based solar cells (first-generation
solar cells). This means that most solar cell manufacturers are
equipped to produce these type of solar cells. Therefore, a large body
of research is currently being done all over the world to create
silicon wafer-based solar cells that can achieve higher conversion
efficiency without an exorbitant increase in production cost. The aim
of the research is to achieve the lowest cost per watt solar cell
design that is suitable for commercial production.[vague]
IBM has a
semiconductor wafer reclamation process that uses a specialized pattern
removal technique to repurpose scrap semiconductor wafers to a form
used to manufacture silicon-based solar panels. The new process was
recently awarded the “2007 Most Valuable Pollution Prevention Award”
from The National Pollution Prevention Roundtable (NPPR). [48]
[edit] Manufacturers
- Further information: Silicon shortage
Solar cells are manufactured primarily in Japan, China, Germany, Taiwan and the USA [49]
, though numerous other nations have or are acquiring significant solar
cell production capacity. While technologies are constantly evolving
toward higher efficiencies, the most effective cells for low cost
electrical production are not necessarily those with the highest
efficiency, but those with a balance between low-cost production and
efficiency high enough to minimize area-related balance of systems
cost. Those companies with large scale manufacturing technology for
coating inexpensive substrates may, in fact, ultimately be the lowest
cost net electricity producers, even with cell efficiencies that are
lower than those of single-crystal technologies.




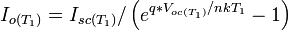
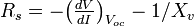



No comments:
Post a Comment